Carbohydrates (CH2O)n
-monomer: glucose
-functions: source of energy, building materials, cell surface makers for cell to cell identification and communication
-divided into three different categories: monosaccharides, polysaccharides, ogliosaccharides
- Monosaccharides contain an aldehyde or a ketone group and one or more hydroxyl groups ( arranged in straight chains while others are branched)
-ogliosaccharides are 2 or 3 simple sugars linked together
-An atom with five carbons is called pentose whereas a carbon with six atoms is referred to as hexose.
-polymers are formed through glycosidic linkages with condensation reactions (H20 is a product)
EXAMPLES: starch and glycogen
Deoxyribonucleic Acids
monomer:nucleotides
functions: inheritance genetics, protein synthesis
chains of units that each consist of a five-carbon sugar, phosphate and a nitrogenous base
polymers are formed through phosphodiester bonds (3 prime of the sugar)
Proteins
Monomer: short sequences of about 157 amino acids
Functions:structural building blocks, functional molecules, enzymes (biological catalysts)
Polymers are formed through peptide bonds
broken down to c terminus and n terminus
c terminus has the carboxyl group while n terminus has the amino group
proteins have four different structures: primary secondary tertiary and quaternary
primary --> simple AA chain,
secondary --> AA chains twirl into helix structure or sheaths,
tertiary--> bending of the chain structure as a result of the attraction of AAs to one another
quaternary--> condensed pack chains of proteins with helix and sheaths
Lipids
hydrocarbons that generally do not dissolve in water but dissolve in non polar substances such as other lipids
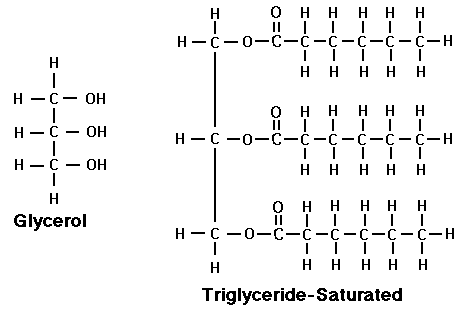
Monomer: glycerol and fatty acid
Functions: storing energy, building membranes (its tail is hydrophobic so it binds with the tail of another phospholipid to create a membrane) and chemical signalling
3 types of lipids: , triglycerides, phospholipids, steroids
triglycerids is a glycerol bonded to three fatty acids through condensation reaction
phospholipids is a glycerol bonded to two fatty acids and a phosphate group
steroids are four interconnecting carbon rings
polymers are created through ester bonds --> condensation reactions
saturated fats are all single bonded hydrogens (with no space available) NOT GOOD cant b decomposed unsaturated have double bond BETTER FAT
E.G. triglyceride,